https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202001452
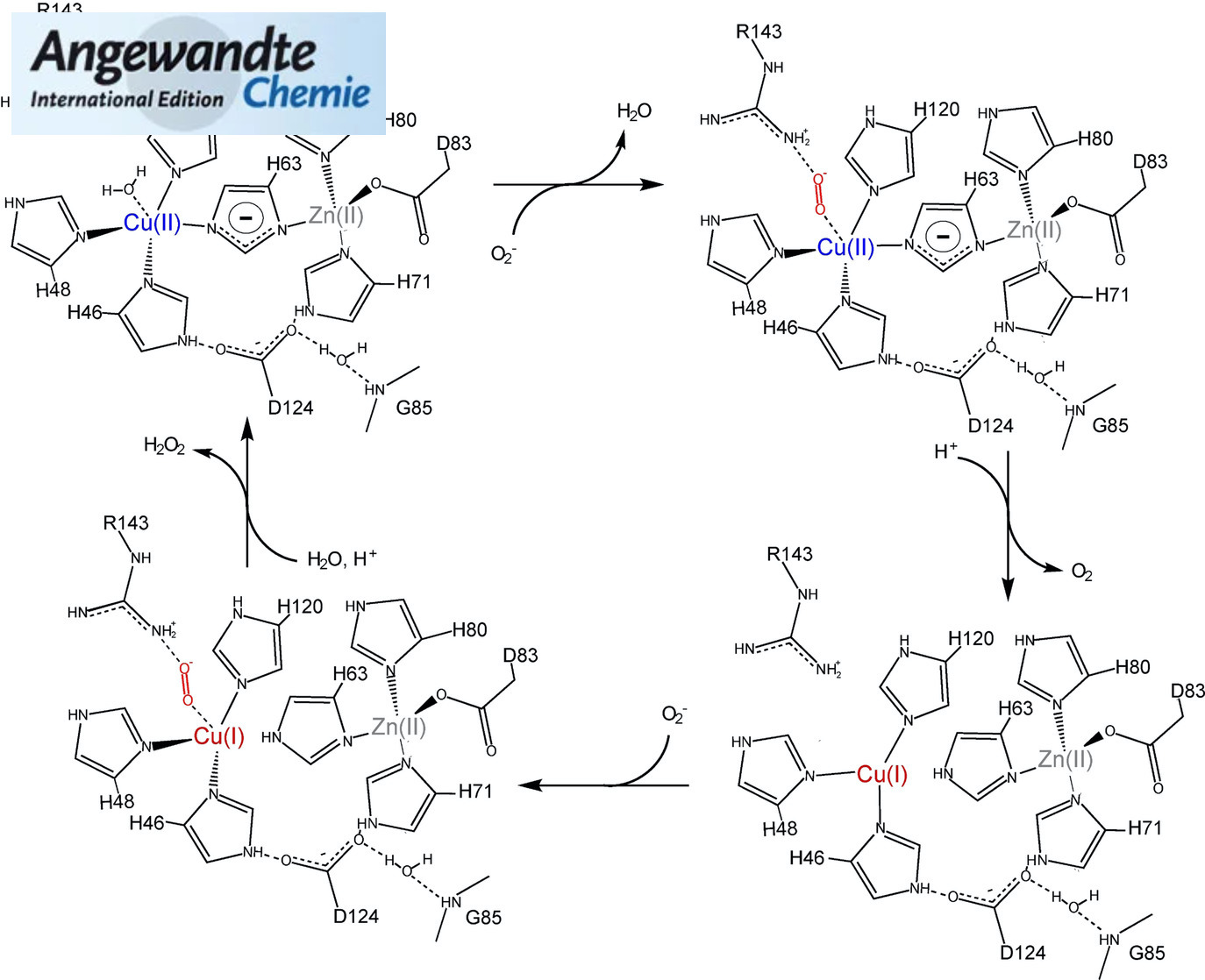
The degree by which metalloproteins partially regulate net charge (Z ) upon electron transfer (ET) was recently measured for the first time using “protein charge ladders” of azurin, cytochrome c, and myoglobin [Angew. Chem. Int. Ed . 2018 , 57 (19), 5364–5368; Angew. Chem . 2018 , 130 , 5462–5466]. Here, we show that Cu, Zn superoxide dismutase (SOD1) is unique among proteins in its ability to resist changes in net charge upon single ET (e.g., ΔZ ET(SOD1)=0.05±0.08 per electron, compared to ΔZ ET(Cyt‐c)=1.19±0.02). This total regulation of net charge by SOD1 is attributed to the protonation of the bridging histidine upon copper reduction, yielding redox centers that are isoelectric at both copper oxidation states. Charge regulation by SOD1 would prevent long range coulombic perturbations to residue pK a’s upon ET at copper, allowing SOD1’s “electrostatic loop” to attract superoxide with equal affinity (at both redox states of copper) during diffusion‐limited reduction and oxidation of superoxide.